Water For Injection Usp Definition

Injectable drugs whose solvent is water.
Water for injection usp definition. Bacteriostatic water for injection bacteriostatic water for injection see usp monograph is sterile water for injection to which has been added one or more suitable antimicrobial preservatives. A non sterile version may be used in manufacturing with. Before such use other substances generally must be added to make the solution more or less isotonic. Sterile water for injection is a pharmaceutic aid vehicle and parenteral fluid replenisher after addition of an appropriate solute.
A sterile version is used for making solutions that will be given by injection. The designation small volume injection applies to an injection that is packaged in containers labeled as containing 100 ml or less. Water for injection usp is chemically designated h 2 o. The usp united states pharmacopeia defines this as highly purified waters containing less than 10 cfu 100 ml of aerobic bacteria.
It is intended to be used as a diluent in the preparation of parenteral products most typically for multi dose products that require repeated. Water for injection by definition is water that is intended for use in the manufacture of parenteral i e. Where used for the preparation of parenteral solutions subject to final sterilization use suitable means to minimize microbial growth or first render the water for injection sterile and thereafter protect it from microbial contamination. Note water for injection is intended for use in the preparation of parenteral solutions.
These are all bulk waters with names given where the pure bulk form of the water is indicated. Usp bacteriostatic water for injection usp sterile water for irrigation the usp designation means that the water is the subject of an official monograph in the current us pharmacopeia with various. It can be given by injection into a vein muscle or under the skin. Containing polypropylene and thermoplastic elastomers free flex.
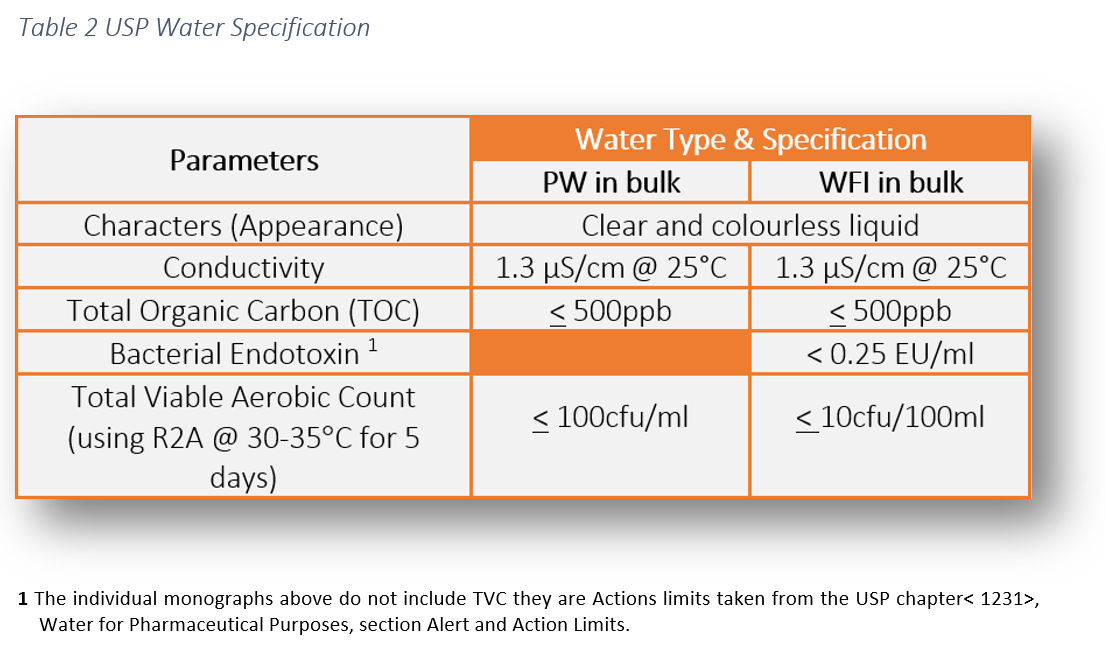
Water for injection is water of extra high quality without significant contamination. Biologics the pharmacopeial definitions for sterile preparations for parenteral use generally do not apply in the case of the biologics because of their special nature and licensing requirements see biologics 1041. Action levels in usp 1231 100cfu ml for purified water and 10cfu 100ml for water for injection are generally considered to represent a level above which the water is unfit for use. That is why an oos investigation must be undertaken if those action levels are exceeded.
For descriptive purposes only. Usp standards for packaged purified water water for injection and sterile purified water usp24 effective 1 1 00 the following are numerical value limits that are commonly used interpretations of the procedures listed on pages 1752 and 1753 under the individual monographs.